Members Login

Channels
Special Offers & Promotions
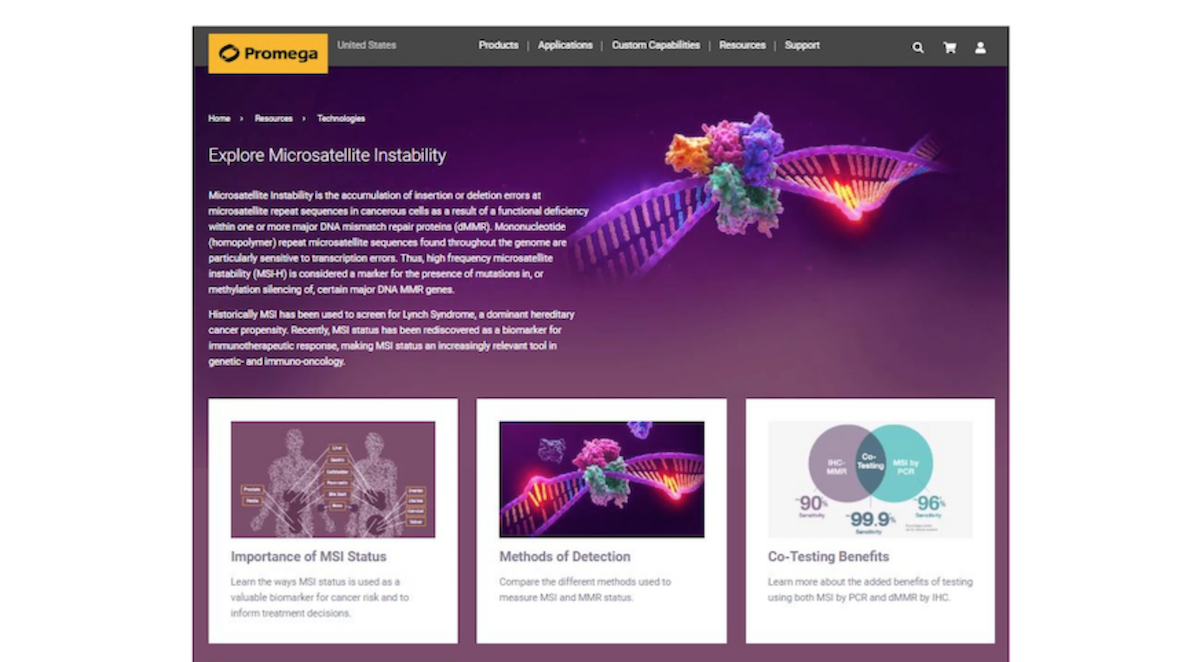
Promega Launches Microsatellite Instability (MSI) Educational Resource
Oncology researchers and clinicians seeking to deepen their understanding of Microsatellite Instability (MSI) have a new resource that gathers a wealth of information in one place, including the importance of MSI as a biomarker and methods of detection.
Promega launched its MSI educational website Promega.com/MSIweb a week ahead of the annual 2019 American Society of Clinical Oncology (ASCO) meeting in Chicago, which is the largest annual worldwide gathering of oncology professionals.
“Education on MSI and similar biomarkers is so important for physicians and patients to understand their treatment options,” says stage IV colorectal cancer survivor and patient consultant Stacy Hurt. “It’s the future of how cancer will be treated.”
Microsatellite Instability is the accumulation of insertion or deletion errors at microsatellite repeat sequences in cancerous cells as a result of a functional deficiency within one or more major DNA mismatch repair proteins (dMMR). Mononucleotide (homopolymer) repeat microsatellite sequences found throughout the genome are particularly sensitive to replication errors. Therefore, the characterisation of a tumour as being Microsatellite Instability-High (MSI-H) is considered a marker for the presence of mutations in, or methylation silencing of, certain major DNA MMR genes.
Historically MSI status and immunohistochemistry (IHC) have been used to screen for Lynch Syndrome, a dominant hereditary cancer propensity. Recently, MSI status has been rediscovered as a biomarker for immunotherapeutic response, with the US Food and Drug Administration making MSI-H status the first tumour agnostic biomarker for the identification of solid tumours appropriate for treatment with Merck’s immunotherapy drug Keytruda® (pembrolizumab).
Recently, the European Society for Medical Oncology recommended the use of both MSI by PCR and MMR by IHC to assess the eligibility of treatment with immune checkpoint inhibitors of metastatic colorectal cancer and other cancers of the Lynch Syndrome spectrum. Co-testing has been shown to be a cost effective and well-established approach to maximising the sensitivity and specificity of MSI and dMMR screen of tumour tissue.
“As biomarkers are being used more and more to guide treatment decisions, education is key,” says Andrew Nixon, Ph.D., MBA, Associate Professor of Medicine at Duke University. “Increased use of validated biomarkers, such as MSI, will ultimately lead to better outcomes for patients.”
Media Partners