Bruker Expands Portfolio for Testing of Candida Auris, an Emerging, Multidrug-Resistant Pathogen in Human Healthcare
New Fungiplex™ Candida Auris PCR Assay for Hygiene and Infection Control (RuO) - New MALDI Biotyper® Diagnostic Workflow for C. Auris Identification
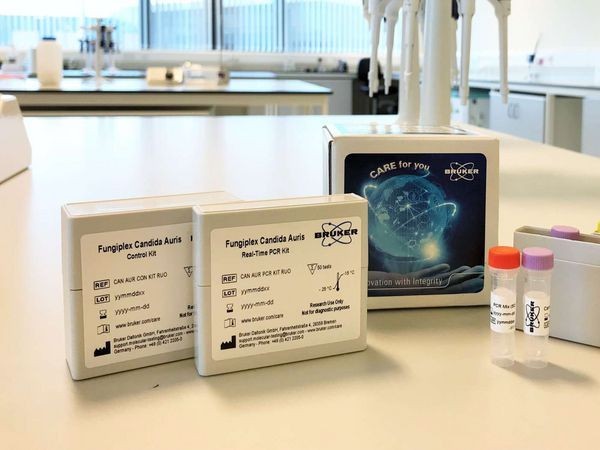
At the International Society for Human and Animal Mycology meeting, Bruker announces Fungiplex™ Candida Auris, a new research-use-only, real-time PCR assay and kit for the detection of Candida auris in hospital hygiene applications.
C. auris is a fungal pathogen capable of causing serious invasive infections in vulnerable patients, which may result in severe illness or death. Often resistant to the most commonly used antifungal drug treatments, it can be transmitted relatively easily between patients, their families and healthcare professional, either directly or through contaminated surfaces or equipment. Outbreaks of C. auris have been observed in hospitals and other healthcare facilities, raising a need to rapidly identify sources of contamination for efficient implementation of infection control measures.
The Fungiplex Candida Auris kit is not intended to be a diagnostic medical device, but is intended to act as an effective epidemiological tool for monitoring hospital environments, and as a research tool allowing investigation of patient colonization. The Fungiplex™ family of tests can be run on multiple real-time PCR platforms under identical conditions, in a user-friendly format, with results reported in less than 2 hours from DNA extraction.
Candida auris is also covered by the comprehensive reference library of Bruker´s market-leading MALDI Biotyper platform for the fast, nearly universal identification of bacterial and fungal species from plate cultures, or from positive blood cultures using the CE-IVD marked Rapid SepsiTyper kit. Bruker recently also has received U.S. FDA clearance for the identification of C. auris from plate cultures on the MALDI Biotyper CA system: See: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm605336.htm
Dr. David Eustace, Head of Infectious Fungal Disease (IFD) detection at Bruker, commented: "The new Fungiplex Candida Auris assay forms an important part of our broadening IFD portfolio. The addition of various culture-free, rapid Fungiplex PCR tests for IFD strengthens the range of options that is now available from Bruker in the fields of infection control and hospital hygiene, alongside the recently released IR Biotyper strain typing system and the industry-leading MALDI Biotyper species ID platform. From rapid detection to in-depth proteomic fingerprinting, Bruker can now address a wide range of customer needs in infection control and hospital hygiene."