Members Login

Channels
Special Offers & Promotions
Advances in 3D Cell Culture
By Cindy Neeley, PhD., Field Applications Scientist, Labware & Speciality Plastics at Thermo Fisher Scientific.
Introduction
![]() Since their introduction in the 80s, three-dimensional (3D) cell cultures have been employed by tissue engineers, cell biologists, and cancer and stem cell scientists, mostly at university research laboratories. The continuous development of materials and cell culture methodologies has enabled the establishment of cell culture conditions that more closely reproduce the environment found in vivo. The first 3D culture methods were cumbersome, time-consuming, and difficult to adapt to routine procedures. Nowadays there are a number of convenient options, from simple to specialized, that can be adapted to fit many specific applications.
Since their introduction in the 80s, three-dimensional (3D) cell cultures have been employed by tissue engineers, cell biologists, and cancer and stem cell scientists, mostly at university research laboratories. The continuous development of materials and cell culture methodologies has enabled the establishment of cell culture conditions that more closely reproduce the environment found in vivo. The first 3D culture methods were cumbersome, time-consuming, and difficult to adapt to routine procedures. Nowadays there are a number of convenient options, from simple to specialized, that can be adapted to fit many specific applications.
Technologies promoting cell self-assembly into 3D spheroid microtissues provide useful culture models and are becoming increasingly popular, especially in fields such as stem cell research and cancer biology. The recent introduction of a new, commercially available polymer-coated surfaces with extremely low cell-binding properties allows the growth of many different cell types into uniform spheroids in suspension.
3D vs 2D culture environment
Most typically, cells are grown in a two-dimensional (2D) environment on dishes of treated polystyrene, where many different types of cell lines can establish uniform monolayers. In a 2D format, the cells grow at a uniform rate due to equal exposure to oxygen, nutrients, additives, and waste products. This approach offers a well-controlled environment, and is much more amenable to maintenance, manipulation and study than the original forms of culture containing pieces of tissue immersed in physiological fluid. Although 2D cultures on plates have revolutionized cellular and molecular biology, 2D cells are spread flat on a plastic surface, and represent a limited model for complex body tissues. Because of this, there have always been research efforts to develop systems that combine the physiological relevance of 3D methods with the convenience of 2D formats.
An important factor in 3D cell culture is the replication of the extracellular matrix (ECM), a structure secreted by cells that provide structural and biochemical support, promoting cell adhesion, cellular communication, and cues for differentiation. The choice of 3D culture format centers around the type of ECM composition, structure and density appropriate for the target tissue, and whether or not to replicate ECM at all. Some 3D culture methods rely on the cells to secrete their own ECM, while others utilize natural or artificial materials to mimic the ECM, until cells create their own.
3D culture formats on solid supports
Many of the platforms commercially available for 3D cell culture are based on the use of solid supports, and can be classified based on the type, geometry and rigidity of the material used.
Gels have a consistency similar to most soft tissues, and somewhat mimic the ECM. They are widely popular due to the availability of many natural and synthetic choices, and because they easily allow the conversion of cultures to 3D format. They can also be combined with other 3D culture methods, like scaffolds and microchips. Gels made from extracts of natural origin, like collagen or alginate have been used for decades and have enabled important discoveries, especially in cancer research. However, many gels for 3D culture are difficult to use due to the gelling mechanism, and are susceptible of uncontrolled structural changes in response to the cells in culture. Gels made from animal origin, although rich in components that stimulate 3D growth, contain undefined constituents (which may include viral agents), and suffer from batch-to-batch variations that make it difficult to interpret and reproduce experimental results.
Scaffolds, or 3D matrices, offer a range of biochemical and mechanical properties designed to model theECM of specific tissues. They are available in a wide range of materials (ceramics, metals, composites, and natural or synthetic polymers), and can be manufactured by a number of techniques, like electrospinning or 3D printing, generating a range of properties suitable for specific applications. Besides the overall size and geometry of the scaffold, many properties of the materials of choice can be considered and manipulated, including biocompatibility, wettability, mechanical properties, surface chemistry, and surface topography (pore interconnectivity, density, size and geometry). While scaffolds offer a wide range of applications and can easily promote 3D growth without sophisticated optimization of cell culture conditions, extraction of cells for analysis can be difficult, and imaging may also become cumbersome.
Microchips, also called “organ on a chip”, are the latest wave of 3D culture models on solid support. This format combines microdevices fabricated with techniques from the microchip industry, with microfluidics technology, allowing 3D cell growth. They aim to reproduce key architectural, biochemical and mechanical features of living organs (like lung, liver, kidney, bone, brain), with the ultimate goal of linking them together and reproducing a complete organism. Despite their unique potential, many of the current systems require considerable expertise to operate. The minute scale involved makes collection and analysis difficult, and high-throughput is currently limited.
3D culture formats for the growth of cells as spheroids in suspension
Although approaches like the ones described above may be necessary for cell lines that depend on solid support for tridimensional growth, these formats suffer from limitations in terms of scalability, reproducibility, sensitivity, and compatibility with high-throughput screening (HTS) instruments.
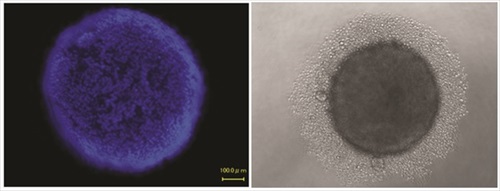
Spheroids, first documented in 1944 by Johannes Holtfreter, are self-assembled, spherical clusters of cells that are able to generate and organize their own ECM. Although only a limited range of cell lines can develop spheroids, they are of great importance in biomedical research, as they mimic some tissues of high relevance in medicine, like avascular solid tumors and stem cell environments. Because spheroids have their own proliferative and metabolic gradients (oxygen, carbon dioxide, nutrients, waste), they constitute excellent physiologic models. In addition, co-culture with other cell types (i.e., endothelial, stromal, or epithelial cells), can expand the applications of this 3D cell culture model.
Spheroids are generated in formats that offer a liquid medium to the cells, but no support structure or porous surfaces, and no added biomaterials or ECM. They can be produced in standardized, scalable way, and are used in both basic laboratory research and HTS applications. The commercial platforms currently available are simple to use, and designed to enable consistent formation of spheroids. They offer simplified liquid handling procedures and compatibility with HTS instruments. The spheroids can be harvested for analysis, or analyzed in microplates by colorimetric, fluorescence, or luminescence assay plate readers. Imaging can also be performed directly in the plate assemblies.
A new development in spheroid culture is the commercial release of culture vessels made of a polymer-coated surface of extremely low binding properties that inhibits adsorption of ECM proteins and cell adhesion, while promoting cell-cell interaction and spheroid formation. The use of this material bypasses the need to establish hanging drops to establish the culture of spheroids. It allows the formation of consistent cancer spheroids in vitro that mimic 3D tumor structures, providing a better model system for the study of tumor progression and the efficacy testing of anticancer agents. It also supports the formation of embryoid bodies and stem cells spheroids of superior quality, with consistent capability to differentiate into all three germ layers, and minimal spontaneous differentiation. It can have robust applications in research involving the differentiation of pluripotent stem cells, where a consistent, reproducible surface is critical to prevent the loss of valuable cells to surface attachment.
Culture vessels made with this material have a long shelf-life and are non-pyrogenic and biologically inert. They come ready to use and in multiple formats (flasks, single or multi-well dishes, round and flat-bottom 96-well plates), for a wide variety of applications. Because they have the standard geometries used for mainstream 2D-culture, their use can be easily integrated into the usual cell culture workflows in the lab, allowing simple operations at all levels, from routine passage of cells to high throughput imaging analysis.
Conclusion
3D culture technologies have expanded our understanding of cellular behavior, but their adoption for applications like cell-based high throughput drug screening, cancer biology, and stem cell research has been slow due to issues of consistency, scale, and cost. New 3D culture formats based on improved materials promise to overcome this limitations and increase its level of applicability.Author
The article was written By Cindy Neeley, PhD. Cindy is a Field Applications Scientists working with Labware & Speciality Plastics at Thermo Fisher Scientific.
more about Thermo Fisher Scientific
more news from Thermo Fisher Scientific
Media Partners