Channels
Special Offers & Promotions
Cherwell launches Redipor® Beta Bags to support continuous manufacturing of sterile medicinal products
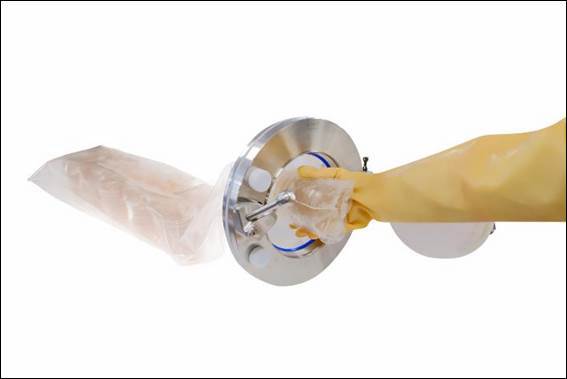
New multiple-use sterile media transfer bags reduce time and risk during product transfer - optimising productivity in pharmaceutical production
Cherwell, cleanroom microbiology solutions expert, has launched the Redipor® Beta Bag to ensure the safe transfer of ready-to-use gamma-irradiated prepared media into sterile manufacturing environments. This new multiple-use product transfer bag can reduce risk, cost and time in environmental monitoring (EM) processes during continuous manufacturing of sterile medicinal products.
Offered as a tailored solution, customers can specify the media, formulation and plate type contained within Redipor Beta Bags, depending on their sterile workflow needs. The new transfer bags are fully validated and compatible with 190mm Getinge Alpha Ports used in isolators and RABS, enabling contamination-free transfer of the Redipor plated media to maintain rapid production and significantly reduce risk.
As the pharmacopoeia compliant media is gamma-irradiated within Redipor Beta Bags, this eliminates the time-consuming decontamination stage required during the standard transfer process of plated media into a sterile workspace for EM. Also streamlining processes, the bags can be attached to a Getinge Alpha Port up to four times without compromising integrity. Sleeves of plates can be stored in the bag and introduced as required, enabling continuous EM, freeing up isolator workspace, and minimising waste of plates and use of single-use plastic packaging.
“We developed our new Redipor Beta Bag to simplify our customers’ sterile workflows during EM programmes for their isolators, whilst optimising safety and efficiency,” said Andy Whittard, Managing Director, Cherwell. “To achieve this, we focused on three key factors during our design process: the contamination-free transfer of Redipor prepared sterile media; time and cost savings for our customers’ processes; and ease-of-use.”
Andy added, “Annex 1 is driving further risk reduction within the pharmaceutical sector. Product innovations, such as our Redipor Beta Bag range, will be needed to keep pace with these changing needs and give our customers a competitive advantage. This new product also supports sustainability initiatives as it reduces need for single-use plastic, use of harmful VHP, and unused plate wastage.”
The Company has created a short video to demonstrate how the Redipor Beta Bag truly simplifies customers’ workflows, giving processes greater adaptability, while ensuring maximum safety of products manufactured, and ultimately patients.
Cherwell provides a comprehensive range of Redipor prepared media options, with room temperature stability and long shelf-life, specifically designed for EM in controlled environments. The new Redipor Beta Bag, which is available with a GS1-compliant data matrix barcode, is a versatile addition to this well-established range, ensuring that the highest levels of production and patient safety is achieved.
About Cherwell Laboratories
Located in Bicester, central England, Cherwell Laboratories provides standard and bespoke cleanroom microbiology solutions that help customers effectively manage their controlled environments and processes. Established for over 50 years, the company has built a strong reputation as a leading supplier of prepared microbiological media and environmental monitoring equipment, with considerable expertise within the pharmaceutical and related industries.
Cherwell manufactures its extensive range of Redipor® prepared media products in its ISO9001 registered facility, and has the flexibility to respond to varying customer requirements - from routine high volume down to low volume specialist applications. In-house engineering facilities support production and the development of bespoke solutions, as well as the supply, service and calibration of its environmental air monitoring equipment solutions.
Cherwell builds strong partnerships through professional, responsive and open communication, and by delivering high quality products, plus a flexible and reliable service with expert support, Cherwell ultimately helps its customers to reduce risk.
Cherwell is now part of the AnalytiChem Group, a collection of eight dynamic businesses located across North America, Europe and Australia.
Recent news from Cherwell Laboratories
Media Partners


