Channels
Special Offers & Promotions
Managing and Maintaining Sterile Manufacturing with Environmental Monitoring LIMS
Following the recent success of our latest webinar Managing and Maintaining Sterile Manufacturing with Environmental Monitoring, Autoscribe Informatics has made the recording available for Quality Management personnel and other interested parties.
Describing the use of the integrated Environmental Monitoring (EM) module as part of Autoscribe’s Matrix Gemini Laboratory Information Management (LIMS) solution, the webinar describes how the module lets quality control teams monitor and manage environmental manufacturing conditions in the pharmaceutical, medical device and food/beverage industries.
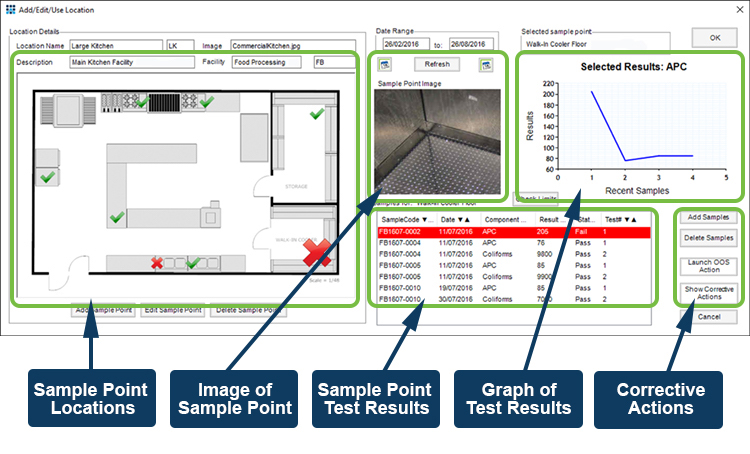
Manufacturing QA/QC in these industries requires constant monitoring of key control points, such as air filters, machine surfaces, touch points such as handles, and product storage areas for contamination. Both microbiological and particulate monitoring may be required.
The EM module allows repeat testing of control points to identify potential contamination. Collected over time, test results can reveal trends that provide an early warning of potential issues; allowing preventative actions to be taken well before contamination causes problems with the product or forces a product recall.
Built-in corrective and preventive action screens provide clear documentation of issues identified, and preventative measures taken to overcome them, giving a complete audit trail for quality inspection teams.
The webinar discusses:
- The importance of Environmental Monitoring to manufacturers
- Why Environmental Monitoring software is important
- Choosing the best solution for your environment
- Using trend analysis to trigger preventative actions
- The risks to avoid in choosing Environmental Monitoring solutions
The Autoscribe Environmental Monitoring module encapsulates the regular repeat testing required for controlled manufacturing. By identifying location and sampling schedules it helps ensure that technicians collect the correct samples from the correct locations at the correct time. This reduces the potential for missed samples and potentially expensive quality failures.
Product recalls in the food industry, in particular, have put a sharp focus on foodborne contamination. This has led manufacturers to introduce preventative sample testing to measure and avoid the root cause of contamination incidents and reap the financial benefits of higher quality production.
Even in organisations that outsource some, or all, of their testing the Autoscribe EM module is a valuable tool for ensuring product quality. Sampling regimes can be managed to ensure the correct samples are taken and once analysed by a third party, results can be imported into the EM providing the same benefits as described above.
Recent news from Autoscribe Informatics
Media Partners


