Channels
Special Offers & Promotions
Autoscribe Release New and Improved Pharmaceutical/Manufacturing-Specific LIMS Solution
Autoscribe Informatics, a leading global laboratory informatics provider, announced the release of a new and improved Matrix Gemini Pharmaceutical/Manufacturing LIMS to meet the changing needs of the pharmaceutical, manufacturing and regulated industries.
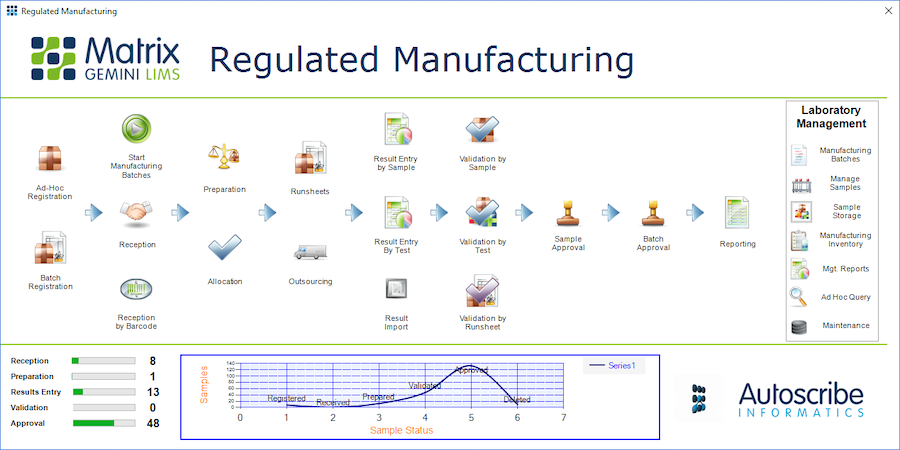
Designed specifically for pharmaceutical and other regulated and non-regulated manufacturing industries the solution allows organisations to track the full batch manufacturing cycle. Full batch traceability allows the tracking of all raw materials and intermediate products used in the process to be linked to the final product. The goal of the solution is to reduce cost, reduce risk and decrease the time required for batch release.
Building on more than 30 years of experience in the LIMS industry the system is built on the standard Matrix Gemini software, configured to the specific needs of pharmaceutical, manufacturing and regulated industries using the Matrix configuration tools. Uniquely, this configuration is separated from the underlying standard code with the benefit that upgrading and re-validation of the software is more straight forward. This leads to a longer system life and lower overall cost of ownership. The Pharmaceutical/Manufacturing specific solution adds to Autoscribe’s range of industry specific LIMS solutions.
“In addition to all the usual sample tracking and testing functions found in a LIMS the pharmaceutical and manufacturing LIMS requires the ability to link batches of raw materials and intermediate products to final products. As well as batch genealogy the system supports many other manufacturing specific features including specification and recipe management, supplier and materials management and customer or market specific certificate of analysis generation”, said Simon Wood, Product Manager of Autoscribe Informatics.
“The improved Pharmaceutical/Manufacturing LIMS brings together, and builds on, many of the features of our existing LIMS solution to provide a comprehensive system for the manufacturing sector.” said Jason Jones, Technical Sales Manager of Autoscribe Informatics. “There are obvious benefits, both from a traceability point of view and a time and financial point of view, to this solution. The goal is to reduce cost, reduce risk and improve operational efficiency overall. It drives faster ‘go live’ timescales, enables easier validation and reduces project risks by encapsulating and reusing best practice”
more news from Autoscribe Informatics
Media Partners