Channels
Special Offers & Promotions
EKF Diagnostics
Products
Contact EKF Diagnostics
All articles from EKF Diagnostics
EKF opens larger facility to increase production of key component for COVID-19 testing regime
Feb 9, 2021
EKF introduces novel molecular transport media for dual COVID-19 and influenza sampling
Jul 15, 2020
Diabetic Ketoacidosis Assay Collaboration Between Ortho Clinical Diagnostics and EKF Diagnostics
Oct 23, 2017
Diabetes Research Unit Cymru Confirms EKF POCT HbA1c Testing Comparable to Lab-Based HPLC
Jul 7, 2017
EKF Showcases New Connectivity Solution for Point-of-Care HbA1c Analyzers at Medica 2016
Nov 6, 2016
New Data Published on PointMan DNA Enrichment Technology for Highly Sensitive Lung Cancer Mutation Detection
Oct 8, 2015
New Four-Way Collaboration Aims to Improve Clinical Decision-Making in the Treatment of Colon Cancer
Mar 5, 2015
EKF Molecular and Massachusetts General Hospital Announce Circulating Tumour Cell Clinical Research Collaboration
Oct 13, 2014
New Data Using EKF PointMan Technology Suggest the Possibility of a Simple Blood Test for Cancers
Jul 30, 2014
Media Partners


 EKF Diagnostics, the global in vitro diagnostics company, announces that it has expanded into new larger manufacturing facilities at Llandough Trading Estate, Cardiff, to increase production of PrimeStore® MTM viral transport media, a key component for the COVID-19 testing regime. The new facility is over 600 sq. metres, representing a 100% increase in manufacturing area. This enables EKF to fill 36,000 PrimeStore MTM tubes daily and meet growing UK and EU demand for this...
EKF Diagnostics, the global in vitro diagnostics company, announces that it has expanded into new larger manufacturing facilities at Llandough Trading Estate, Cardiff, to increase production of PrimeStore® MTM viral transport media, a key component for the COVID-19 testing regime. The new facility is over 600 sq. metres, representing a 100% increase in manufacturing area. This enables EKF to fill 36,000 PrimeStore MTM tubes daily and meet growing UK and EU demand for this...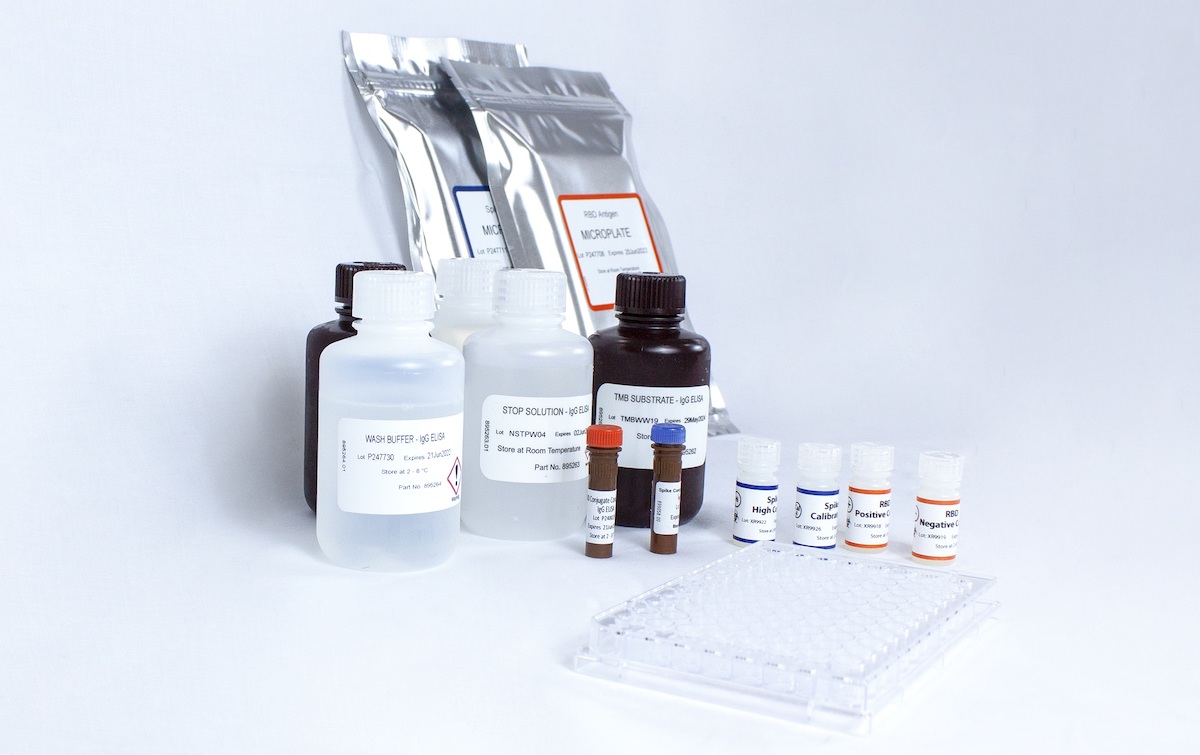 EKF Diagnostics, the global in vitro diagnostics company, announces that it has introduced one of the first tests to precisely measure levels of COVID-19 neutralizing antibodies in individuals. Unlike other antibody tests, the Kantaro COVID-SeroKlir SARS-CoV-2 IgG antibody test kit determines both the presence and specific quantities of human IgG antibodies to the SARS-CoV-2 virus. This enables a broad range of COVID-19 applications...
EKF Diagnostics, the global in vitro diagnostics company, announces that it has introduced one of the first tests to precisely measure levels of COVID-19 neutralizing antibodies in individuals. Unlike other antibody tests, the Kantaro COVID-SeroKlir SARS-CoV-2 IgG antibody test kit determines both the presence and specific quantities of human IgG antibodies to the SARS-CoV-2 virus. This enables a broad range of COVID-19 applications...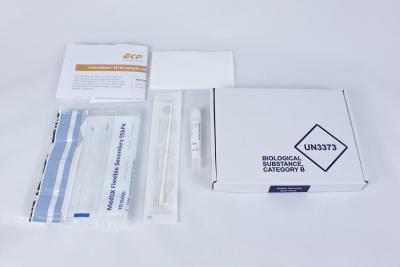 EKF Diagnostics, the global in vitro diagnostics company, announces that it has launched a PrimeStore® MTM sample collection kit. This new kit enables convenient and safe collection, transportation and handling of pathogenic samples, including COVID-19 and influenza. Two kit configurations are now available: a postal sample kit ideal for home use and a multi-pack of sample kits suitable for on-site mass sampling....
EKF Diagnostics, the global in vitro diagnostics company, announces that it has launched a PrimeStore® MTM sample collection kit. This new kit enables convenient and safe collection, transportation and handling of pathogenic samples, including COVID-19 and influenza. Two kit configurations are now available: a postal sample kit ideal for home use and a multi-pack of sample kits suitable for on-site mass sampling.... 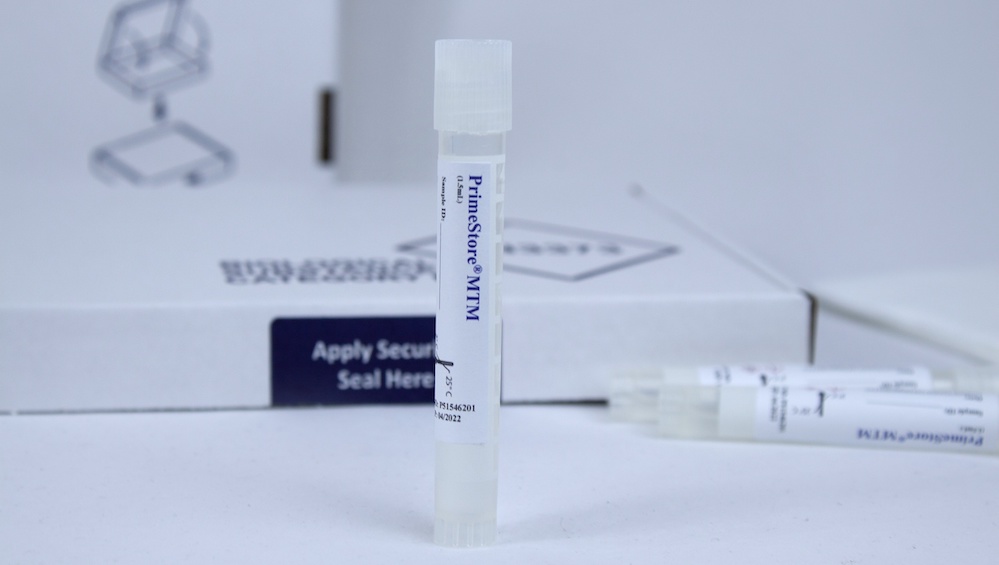 EKF Diagnostics, the global in vitro diagnostics company, announces that the novel viral transport media PrimeStore® MTM (Molecular Transport Medium) has been successfully evaluated for effective SARS-CoV-2 inactivation in a new study published by Public Health England (PHE). To work safely with live SARS-CoV-2 samples requires the use of high-containment laboratories....
EKF Diagnostics, the global in vitro diagnostics company, announces that the novel viral transport media PrimeStore® MTM (Molecular Transport Medium) has been successfully evaluated for effective SARS-CoV-2 inactivation in a new study published by Public Health England (PHE). To work safely with live SARS-CoV-2 samples requires the use of high-containment laboratories.... EKF Diagnostics, the global in vitro diagnostics company, announces that it has added a novel viral transport media for the safe sample handling and testing of multiple infectious diseases from a single swab to its product range. PrimeStore® MTM (Molecular Transport Medium) is an FDA cleared and CE IVD marked sample collection device which deactivates viruses, including COVID-19, flu A, flu B, HIV and TB....
EKF Diagnostics, the global in vitro diagnostics company, announces that it has added a novel viral transport media for the safe sample handling and testing of multiple infectious diseases from a single swab to its product range. PrimeStore® MTM (Molecular Transport Medium) is an FDA cleared and CE IVD marked sample collection device which deactivates viruses, including COVID-19, flu A, flu B, HIV and TB....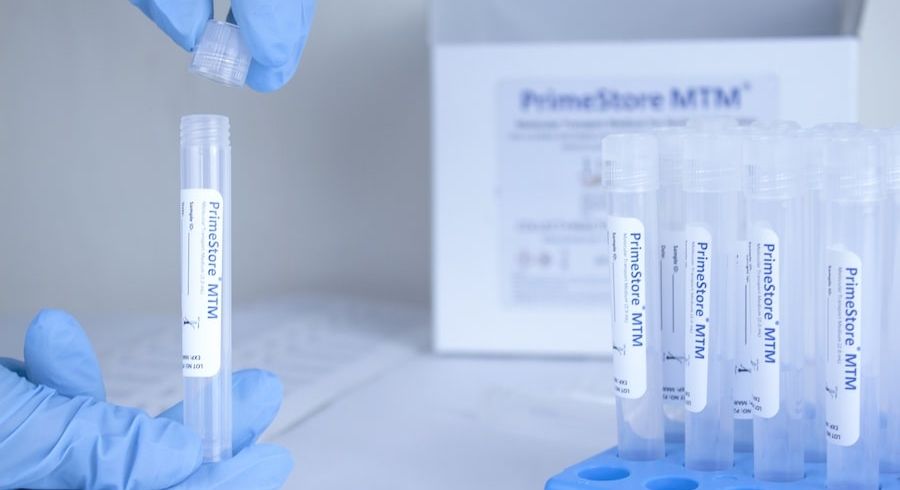 EKF Diagnostics, the global in vitro diagnostics company, announces it has secured new contracts for the manufacture and supply of a novel, patented sample collection device which allows COVID-19 samples to be rapidly inactivated in the collection tube, avoiding contamination and preserving RNA without need for refrigeration. To support the increase in COVID-19 testing globally, a safe and easy sample collection and transport mechanism is essential, this is provided by the PrimeStore® MTM sample collection device....
EKF Diagnostics, the global in vitro diagnostics company, announces it has secured new contracts for the manufacture and supply of a novel, patented sample collection device which allows COVID-19 samples to be rapidly inactivated in the collection tube, avoiding contamination and preserving RNA without need for refrigeration. To support the increase in COVID-19 testing globally, a safe and easy sample collection and transport mechanism is essential, this is provided by the PrimeStore® MTM sample collection device.... EKF Diagnostics, the global in vitro diagnostics company, announces that it has opened a new Research & Development facility at its Cardiff, UK headquarters. The new Special Projects Laboratory will focus on developing new applications for EKF’s many point-of-care and central laboratory medical products to enable the Company to enter new market sectors, as well as providing additional diagnostics sales and regulatory support....
EKF Diagnostics, the global in vitro diagnostics company, announces that it has opened a new Research & Development facility at its Cardiff, UK headquarters. The new Special Projects Laboratory will focus on developing new applications for EKF’s many point-of-care and central laboratory medical products to enable the Company to enter new market sectors, as well as providing additional diagnostics sales and regulatory support....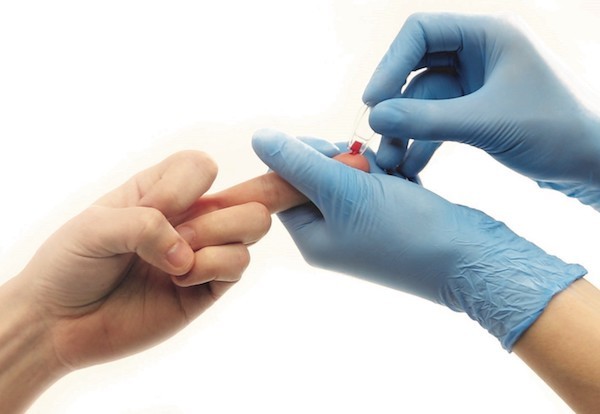 Incorrect capillary blood sampling is most common reason for inaccurate POC hemoglobin results.
Incorrect capillary blood sampling is most common reason for inaccurate POC hemoglobin results. 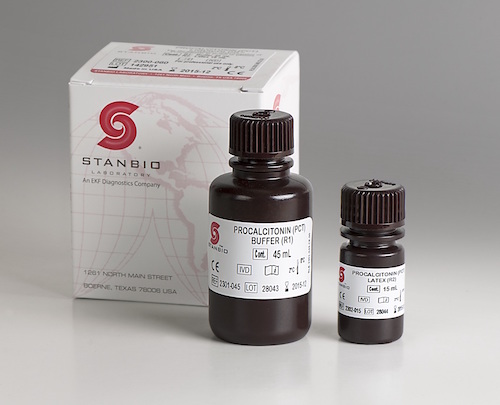 EKF Diagnostics, the global in vitro diagnostics company, announces that its Stanbio Chemistry Procalcitonin (PCT) LiquiColor® assay has been FDA cleared and validated for use on Beckman AU 480, 680 and 5800 clinical chemistry analyzers.....
EKF Diagnostics, the global in vitro diagnostics company, announces that its Stanbio Chemistry Procalcitonin (PCT) LiquiColor® assay has been FDA cleared and validated for use on Beckman AU 480, 680 and 5800 clinical chemistry analyzers.....
 EKF Diagnostics, the global in vitro diagnostics company, has published a guide to ‘Diabetes and HbA1c testing’ which can be found at EKF’s new Diabetes Portal. This new educational guide draws on EKF’s expertise in the diagnosis and monitoring of diabetes and associated conditions....
EKF Diagnostics, the global in vitro diagnostics company, has published a guide to ‘Diabetes and HbA1c testing’ which can be found at EKF’s new Diabetes Portal. This new educational guide draws on EKF’s expertise in the diagnosis and monitoring of diabetes and associated conditions....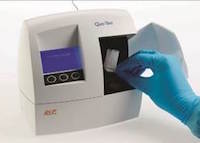 EKF Diagnostics, the global in vitro diagnostics company, announces that a recent study has confirmed that its Quo-Test® A1c point-of-care testing (POCT) analyzer shows comparable performance to a lab-based HPLC system for the measurement of glycated hemoglobin (HbA1c)....
EKF Diagnostics, the global in vitro diagnostics company, announces that a recent study has confirmed that its Quo-Test® A1c point-of-care testing (POCT) analyzer shows comparable performance to a lab-based HPLC system for the measurement of glycated hemoglobin (HbA1c).... EKF Diagnostics, the global in vitro diagnostics company, announces that it is expanding the distribution of its Procalcitonin LiquiColor® Test into Eastern Europe, Middle East and APAC regions. Procalcitonin (PCT) is a marker for bacterial infection and sepsis, a condition that has grown in awareness in recent years....
EKF Diagnostics, the global in vitro diagnostics company, announces that it is expanding the distribution of its Procalcitonin LiquiColor® Test into Eastern Europe, Middle East and APAC regions. Procalcitonin (PCT) is a marker for bacterial infection and sepsis, a condition that has grown in awareness in recent years.... EKF Diagnostics, the global in vitro diagnostics company, announces that in a newly published paper its Quo-Test® point-of-care (POC) hemoglobin A1c (HbA1c) analyzer has again been confirmed to meet International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) performance criteria....
EKF Diagnostics, the global in vitro diagnostics company, announces that in a newly published paper its Quo-Test® point-of-care (POC) hemoglobin A1c (HbA1c) analyzer has again been confirmed to meet International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) performance criteria.... EKF Diagnostics, the global in vitro diagnostics company, announces that it will be previewing a new point-of-care (POC) connectivity solution for its range of HbA1c analyzers at Medica 2016 in Düsseldorf from 14–17th November. In Hall 3 / C70, EKF will be showcasing the new connectivity package for Quo-Test™ and Quo-Lab™ HbA1c analyzers...
EKF Diagnostics, the global in vitro diagnostics company, announces that it will be previewing a new point-of-care (POC) connectivity solution for its range of HbA1c analyzers at Medica 2016 in Düsseldorf from 14–17th November. In Hall 3 / C70, EKF will be showcasing the new connectivity package for Quo-Test™ and Quo-Lab™ HbA1c analyzers... EKF Diagnostics, the global in vitro diagnostics company, announces that its newly introduced Glycated Serum Protein (GSP) LiquiColor® diabetic biomarker test has been verified for use on the Siemens Vista chemistry analyzer. In a scientific poster published by scientists at the Memorial Healthcare System, Hollywood, USA [1], it was demonstrated that EKF’s GSP assay enhances the versatility of the Vista system for the specialized glycemic monitoring of...
EKF Diagnostics, the global in vitro diagnostics company, announces that its newly introduced Glycated Serum Protein (GSP) LiquiColor® diabetic biomarker test has been verified for use on the Siemens Vista chemistry analyzer. In a scientific poster published by scientists at the Memorial Healthcare System, Hollywood, USA [1], it was demonstrated that EKF’s GSP assay enhances the versatility of the Vista system for the specialized glycemic monitoring of... EKF Diagnostics
EKF Diagnostics
 EKF Diagnostics, the global in vitro diagnostics company, announces that results from a study involving EKF Molecular’s PointMan™ DNA enrichment technology have been published in Clinical Cancer Research [1], a journal of the American Association for Cancer Research which focusses on innovative clinical cancer research studies. The study comparing tumor biopsy with noninvasive blood-based analyses brings together...
EKF Diagnostics, the global in vitro diagnostics company, announces that results from a study involving EKF Molecular’s PointMan™ DNA enrichment technology have been published in Clinical Cancer Research [1], a journal of the American Association for Cancer Research which focusses on innovative clinical cancer research studies. The study comparing tumor biopsy with noninvasive blood-based analyses brings together... EKF Diagnostics, the global in vitro diagnostics company, announces the international launch of the Altair™ 240 clinical chemistry analyzer at the American Association for Clinical Chemistry’s (AACC) Annual Meeting and Clinical Lab Expo in Atlanta, Ga, 26-30 July. This new bench-top platform represents EKF Diagnostics’ first fully integrated chemistry system designed for the global market. Also on Booth #1018, EKF will highlight the latest additions to its range of...
EKF Diagnostics, the global in vitro diagnostics company, announces the international launch of the Altair™ 240 clinical chemistry analyzer at the American Association for Clinical Chemistry’s (AACC) Annual Meeting and Clinical Lab Expo in Atlanta, Ga, 26-30 July. This new bench-top platform represents EKF Diagnostics’ first fully integrated chemistry system designed for the global market. Also on Booth #1018, EKF will highlight the latest additions to its range of... EKF Diagnostics
EKF Diagnostics This new test enables the quantitative determination of PCT in serum samples, EDTA or lithium heparin plasma samples by latex enhanced immunoturbidimetric methodology. Procalcitonin is a marker for bacterial infection and sepsis and has been recognized as an important adjunct marker in the diagnosis of sepsis*. The new Stanbio Chemistry Procalcitonin (PCT) LiquiColor® Assay is fast, accurate and convenient. The test provides...
This new test enables the quantitative determination of PCT in serum samples, EDTA or lithium heparin plasma samples by latex enhanced immunoturbidimetric methodology. Procalcitonin is a marker for bacterial infection and sepsis and has been recognized as an important adjunct marker in the diagnosis of sepsis*. The new Stanbio Chemistry Procalcitonin (PCT) LiquiColor® Assay is fast, accurate and convenient. The test provides... EKF Diagnostics, the global diagnostics company, announces that its PointMan™ DNA enrichment technology has been identified as an easy and useful method for non-invasively determining plasma EGFR T790M mutation status in late stage lung cancer patients. This is according to data presented at the American Association for Cancer Research (AACR) Annual Meeting 2015, April 18 - 22, 2015, Philadelphia, Pennsylvania. In a scientific poster entitled...
EKF Diagnostics, the global diagnostics company, announces that its PointMan™ DNA enrichment technology has been identified as an easy and useful method for non-invasively determining plasma EGFR T790M mutation status in late stage lung cancer patients. This is according to data presented at the American Association for Cancer Research (AACR) Annual Meeting 2015, April 18 - 22, 2015, Philadelphia, Pennsylvania. In a scientific poster entitled... EKF Diagnostics subsidiary, Selah Genomics, has announced a major, four-way collaboration with Greenville Health System (GHS, South Carolina), DecisionQ Corporation (Virginia), and BD (Becton Dickinson and Company, New Jersey). Expected to last 18 months, the collaboration aims to unite classic clinical annotations with proprietary next generation sequencing (NGS) technology and artificial intelligence-based decision...
EKF Diagnostics subsidiary, Selah Genomics, has announced a major, four-way collaboration with Greenville Health System (GHS, South Carolina), DecisionQ Corporation (Virginia), and BD (Becton Dickinson and Company, New Jersey). Expected to last 18 months, the collaboration aims to unite classic clinical annotations with proprietary next generation sequencing (NGS) technology and artificial intelligence-based decision... EKF Diagnostics
EKF Diagnostics
 EKF Diagnostics, the global diagnostics company, announces that it has entered into a two year research collaboration with Massachusetts General Hospital (MGH), a global leader in successfully bridging innovative science with state-of-the-art clinical medicine, to develop PointMan™ assays that can effectively detect treatable cancer mutations in blood samples. The collaboration agreement has been signed following a detailed evaluation of...
EKF Diagnostics, the global diagnostics company, announces that it has entered into a two year research collaboration with Massachusetts General Hospital (MGH), a global leader in successfully bridging innovative science with state-of-the-art clinical medicine, to develop PointMan™ assays that can effectively detect treatable cancer mutations in blood samples. The collaboration agreement has been signed following a detailed evaluation of... This is significant since with such sensitivity, it paves the way for the possibility of using a simple blood test to screen and diagnose different cancers, as well as monitor the efficacy of anti-cancer therapies. Following work undertaken by the Swansea-based team earlier this year, which successfully detected circulating free DNA (cfDNA) mutations from melanoma patients using PointMan, further studies were carried out on...
This is significant since with such sensitivity, it paves the way for the possibility of using a simple blood test to screen and diagnose different cancers, as well as monitor the efficacy of anti-cancer therapies. Following work undertaken by the Swansea-based team earlier this year, which successfully detected circulating free DNA (cfDNA) mutations from melanoma patients using PointMan, further studies were carried out on... Dr. Andrzej Krolewski, MD, PhD, Head of Section on Genetics and Epidemiology at the Joslin Diabetes Center and Professor of Medicine at Harvard Medical School, receives this eminent award in recognition of his significant contribution to the field of diabetes epidemiology. At the ADA 74th Scientific Sessions (13-17th June, 2014, San Francisco), Dr. Krolewski will also deliver the Kelly West Award lecture...
Dr. Andrzej Krolewski, MD, PhD, Head of Section on Genetics and Epidemiology at the Joslin Diabetes Center and Professor of Medicine at Harvard Medical School, receives this eminent award in recognition of his significant contribution to the field of diabetes epidemiology. At the ADA 74th Scientific Sessions (13-17th June, 2014, San Francisco), Dr. Krolewski will also deliver the Kelly West Award lecture... EKF affirms that the markers can be reliably used as diagnostic tests to predict end-stage renal disease (ESRD) - one of the greatest mortality risks in diabetics - up to 10 years in advance. Since signing an exclusive license agreement for this novel kidney biomarker technology with the prestigious Joslin Diabetes Center (Boston, USA) in 2012, EKF Diagnostics has worked in partnership with Joslin and other key diabetic research centers to further...
EKF affirms that the markers can be reliably used as diagnostic tests to predict end-stage renal disease (ESRD) - one of the greatest mortality risks in diabetics - up to 10 years in advance. Since signing an exclusive license agreement for this novel kidney biomarker technology with the prestigious Joslin Diabetes Center (Boston, USA) in 2012, EKF Diagnostics has worked in partnership with Joslin and other key diabetic research centers to further... Following the recent launch of its new Lactate Scout+ analyzer incorporating hematocrit compensation, EKF Diagnostics, the global diagnostics business, is seeing an increasing interest from a broad range of users in both sports and medical science. Consequently, EKF has launched a specific product resource website (www.lactatescout.com) housing numerous guides and scientific papers on lactate testing...
Following the recent launch of its new Lactate Scout+ analyzer incorporating hematocrit compensation, EKF Diagnostics, the global diagnostics business, is seeing an increasing interest from a broad range of users in both sports and medical science. Consequently, EKF has launched a specific product resource website (www.lactatescout.com) housing numerous guides and scientific papers on lactate testing... EKF Diagnostics, the global in vitro diagnostics business, announces that it has acquired Separation Technology, Inc. (STI), the Florida based manufacturer of in vitro diagnostics devices for hematology testing. This acquisition complements EKF’s existing offering in the hemoglobin testing market place, which includes Hemo Control (also sold as HemoPoint H2 in USA and Asia). Notably, STI’s primary instrument is the UltraCrit hematocrit measurement device which is FDA cleared for blood donor screening....
EKF Diagnostics, the global in vitro diagnostics business, announces that it has acquired Separation Technology, Inc. (STI), the Florida based manufacturer of in vitro diagnostics devices for hematology testing. This acquisition complements EKF’s existing offering in the hemoglobin testing market place, which includes Hemo Control (also sold as HemoPoint H2 in USA and Asia). Notably, STI’s primary instrument is the UltraCrit hematocrit measurement device which is FDA cleared for blood donor screening.... EKF Diagnostics, the global in vitro diagnostics business, announces a major breakthrough for its PointMan™ DNA enrichment technology for potential use in future cancer testing and treatment. The first successful results of a collaboration between EKF Molecular Diagnostics and the Institute of Life Sciences at Swansea University have demonstrated the detection of gene mutations in blood from samples archived in the Wales Cancer Bank....
EKF Diagnostics, the global in vitro diagnostics business, announces a major breakthrough for its PointMan™ DNA enrichment technology for potential use in future cancer testing and treatment. The first successful results of a collaboration between EKF Molecular Diagnostics and the Institute of Life Sciences at Swansea University have demonstrated the detection of gene mutations in blood from samples archived in the Wales Cancer Bank.... EKF Diagnostics, a global diagnostics company, is pleased to announce that its Quo-Lab HbA1c point-of-care analyzer has successfully achieved International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) certification. The IFCC maintains the JCTLM (Joint Committee for Traceability in Laboratory Medicine) endorsed reference measurement procedure for HbA1c, accepted worldwide as the analytical control for traceability of HbA1c measurement....
EKF Diagnostics, a global diagnostics company, is pleased to announce that its Quo-Lab HbA1c point-of-care analyzer has successfully achieved International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) certification. The IFCC maintains the JCTLM (Joint Committee for Traceability in Laboratory Medicine) endorsed reference measurement procedure for HbA1c, accepted worldwide as the analytical control for traceability of HbA1c measurement....
 EKF Diagnostics, the global diagnostics business, announces the publication of a new white paper detailing the successful external evaluation of its Quo-Test and Quo-Lab glycated hemoglobin (HbA1c) analyzers - ‘Evaluation of four POCT-Systems for the measurement of HbA1c’. The aim of the study by scientists at IMCARMED GmbH was to compare four point-of-care (POCT) methods with established laboratory techniques for the determination of HbA1c, an internationally recognized biomarker of blood glucose levels for diabetes management...
EKF Diagnostics, the global diagnostics business, announces the publication of a new white paper detailing the successful external evaluation of its Quo-Test and Quo-Lab glycated hemoglobin (HbA1c) analyzers - ‘Evaluation of four POCT-Systems for the measurement of HbA1c’. The aim of the study by scientists at IMCARMED GmbH was to compare four point-of-care (POCT) methods with established laboratory techniques for the determination of HbA1c, an internationally recognized biomarker of blood glucose levels for diabetes management...