Channels
Special Offers & Promotions
Copley Scientific Limited
Products
Contact Copley Scientific Limited
All articles from Copley Scientific Limited
Introducing the new NGI Assistant, a Labour-Saving Device for Higher Productivity Inhaler Testing
Aug 15, 2018
Copley Scientific Invests Locally and Boosts Rate of Growth, to Support Growing International Business
Aug 5, 2017
Copley Scientific Celebrates Seven Decades of Responsive Service to the Pharmaceutical Industry
May 24, 2016
Copley Scientific launches new products for MDI testing in line with the new USP monograph
Feb 11, 2014
New media prep station from Copley Scientific increases productivity in tablet dissolution testing
Aug 19, 2013
Copley Scientific introduces the Dissofract, an automated sampler for dissolution testing
Jul 31, 2013
New Copley Scientific super capacity vacuum pump supports high flow rate testing of inhaled products
Nov 15, 2012
Copley Scientific highlights new research tool for aerosol characterisation at EAC 2012
Jul 31, 2012
Copley Scientific delivers eight further dissolution testers to thriving Melbourn Scientific
Mar 10, 2011
Media Partners


 Inhalytix®+ from Copley is an innovative software package that revolutionises cascade impaction workflows by connecting equipment, automating data transfer and centralising analysis in a single validated system. As the global leader in inhaler testing solutions, Copley has deep expertise in the needs of orally inhaled and nasal drug product (OINDP) analysts working in busy labs to deliver data of exemplary quality, as efficiently as possible...
Inhalytix®+ from Copley is an innovative software package that revolutionises cascade impaction workflows by connecting equipment, automating data transfer and centralising analysis in a single validated system. As the global leader in inhaler testing solutions, Copley has deep expertise in the needs of orally inhaled and nasal drug product (OINDP) analysts working in busy labs to deliver data of exemplary quality, as efficiently as possible... EnviroMate™ is a benchtop environmental control chamber from Copley, the world’s leading manufacturer and supplier of inhaler testing equipment, designed specifically to improve the repeatability and integrity of inhaler test data. The performance of orally inhaled and nasal drug products (OINDPs) can be directly influenced by ambient temperature, humidity and electrostatics found in the laboratory, as highlighted by the US and European Pharmacopoeias...
EnviroMate™ is a benchtop environmental control chamber from Copley, the world’s leading manufacturer and supplier of inhaler testing equipment, designed specifically to improve the repeatability and integrity of inhaler test data. The performance of orally inhaled and nasal drug products (OINDPs) can be directly influenced by ambient temperature, humidity and electrostatics found in the laboratory, as highlighted by the US and European Pharmacopoeias... Hands-on demonstrations at Copley Scientific’s UK headquarters are proving the perfect introduction to the upgraded NGI (Next Generation Impactor) Assistant, a labour-saving device that boosts the precision and productivity of inhaler testing to unprecedented levels. Cascade impaction is used to determine the aerodynamic particle size distribution (APSD) of all orally inhaled products; a critical quality attribute....
Hands-on demonstrations at Copley Scientific’s UK headquarters are proving the perfect introduction to the upgraded NGI (Next Generation Impactor) Assistant, a labour-saving device that boosts the precision and productivity of inhaler testing to unprecedented levels. Cascade impaction is used to determine the aerodynamic particle size distribution (APSD) of all orally inhaled products; a critical quality attribute....
 Copley is investing heavily into infrastructure, by expanding its UK HQ to reinforce the company’s export base, which currently supplies to over 90 countries globally. The investment also encompasses recruitment, with the current focus on strengthening customer facing teams....
Copley is investing heavily into infrastructure, by expanding its UK HQ to reinforce the company’s export base, which currently supplies to over 90 countries globally. The investment also encompasses recruitment, with the current focus on strengthening customer facing teams.... The new guide will help all those looking to establish relevant, reproducible, cost-efficient methods for formulation, QC and comparative assessment of detergents. Download it via: http://bit.ly/COPDET. All cleaning products are tested in full scale domestic washing machines and dishwashers, but such testing is time-, energy- and detergent-intensive....
The new guide will help all those looking to establish relevant, reproducible, cost-efficient methods for formulation, QC and comparative assessment of detergents. Download it via: http://bit.ly/COPDET. All cleaning products are tested in full scale domestic washing machines and dishwashers, but such testing is time-, energy- and detergent-intensive....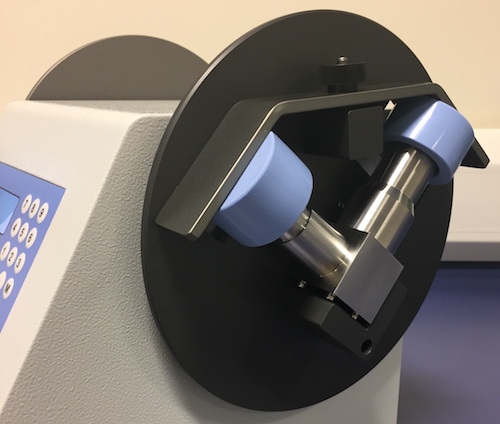 The upgraded SPU 2000 now accommodates a Fluticasone Propionate/Salmeterol Xinafoate (FP/SX) induction port, in addition to the standard Andersen Cascade Impactor (ACI) and Next Generation Impactor (NGI) induction ports, making it a universally applicable labour-saving device for rinsing the induction ports routinely used in cascade impactor testing....
The upgraded SPU 2000 now accommodates a Fluticasone Propionate/Salmeterol Xinafoate (FP/SX) induction port, in addition to the standard Andersen Cascade Impactor (ACI) and Next Generation Impactor (NGI) induction ports, making it a universally applicable labour-saving device for rinsing the induction ports routinely used in cascade impactor testing.... Copley Scientific, a global leader in inhaler test equipment manufacturing, has grown exponentially over recent years and the launch of the Chinese version of the company website is further evidence of the company’s continued global success. The new building is expected to come on stream this year....
Copley Scientific, a global leader in inhaler test equipment manufacturing, has grown exponentially over recent years and the launch of the Chinese version of the company website is further evidence of the company’s continued global success. The new building is expected to come on stream this year.... The videos, entitled ‘How does a cascade impactor work?’ and ‘System for improved in vitro-in vivo correlations (IVIVCs) of inhaled drug products’ are an excellent resource for training, for researchers new to inhaled product testing and/or those seeking enhanced testing strategies...
The videos, entitled ‘How does a cascade impactor work?’ and ‘System for improved in vitro-in vivo correlations (IVIVCs) of inhaled drug products’ are an excellent resource for training, for researchers new to inhaled product testing and/or those seeking enhanced testing strategies... “Longevity in business is a true mark of success and in our case a direct tribute to all the staff who have dedicated so much of their time and energy to the company, over many years.” said Mark Copley, Sales Director for Copley Scientific. “The loyalty of our team is reflected in the stability of our customer base and a primary reason for our outstanding customer service and application expertise. I’d like to thank everyone...
“Longevity in business is a true mark of success and in our case a direct tribute to all the staff who have dedicated so much of their time and energy to the company, over many years.” said Mark Copley, Sales Director for Copley Scientific. “The loyalty of our team is reflected in the stability of our customer base and a primary reason for our outstanding customer service and application expertise. I’d like to thank everyone... Considered a ‘go to’ resource for expert advice that is rooted in current regulatory guidance and pharmacopoeial chapters, the brand new brochure can be freely downloaded from the Copley website, with printed versions available on request from sales@copleyscientific.co.uk. The 2016 brochure covers the latest international requirements for testing the safety, quality and efficacy of transdermals, powders and granules, tablets and capsules, suppositories, creams and ointments...
Considered a ‘go to’ resource for expert advice that is rooted in current regulatory guidance and pharmacopoeial chapters, the brand new brochure can be freely downloaded from the Copley website, with printed versions available on request from sales@copleyscientific.co.uk. The 2016 brochure covers the latest international requirements for testing the safety, quality and efficacy of transdermals, powders and granules, tablets and capsules, suppositories, creams and ointments... Semisolids are creams, ointments, gels or lotions used principally in the treatment of dermatological conditions that are applied topically to the skin to deliver a localised therapeutic response. The performance of such products is determined by measuring the release rate of the active ingredient, through a suitable membrane held in contact with a receptor medium contained within a VDC.
Semisolids are creams, ointments, gels or lotions used principally in the treatment of dermatological conditions that are applied topically to the skin to deliver a localised therapeutic response. The performance of such products is determined by measuring the release rate of the active ingredient, through a suitable membrane held in contact with a receptor medium contained within a VDC.  The scaled down test environment offered by the well-established Tergotometer provides representative testing while using minimal quantities of detergent. The resulting data enables the robust assessment and comparison of detergent performance, to accelerate product development.
The scaled down test environment offered by the well-established Tergotometer provides representative testing while using minimal quantities of detergent. The resulting data enables the robust assessment and comparison of detergent performance, to accelerate product development.  ‘Quality Solutions for Inhaler Testing, 2015 Edition’ includes detailed information on regulatory requirements and highlights pertinent new additions to the company’s extensive inhaled product testing range. It can be downloaded freely from the Copley Scientific website (www.copleyscientific.com) with print versions available on request from sales@copleyscientific.co.uk. “We know that our customers value help with understanding the testing requirements for OINDPs, which...
‘Quality Solutions for Inhaler Testing, 2015 Edition’ includes detailed information on regulatory requirements and highlights pertinent new additions to the company’s extensive inhaled product testing range. It can be downloaded freely from the Copley Scientific website (www.copleyscientific.com) with print versions available on request from sales@copleyscientific.co.uk. “We know that our customers value help with understanding the testing requirements for OINDPs, which... His invited presentation on 23rd February 2015 is part of a 2-day event that will cover all aspects of capsule based DPI technology. The event takes place at the Hotel Sonne and Harro Höfliger Verpackungsmaschinen GmbH headquarters in Germany. Mark Copley’s presentation will provide a basic introduction to the dose uniformity and aerodynamic particle size distribution methods required for the testing of orally inhaled products (OIPs), according to the requirements of...
His invited presentation on 23rd February 2015 is part of a 2-day event that will cover all aspects of capsule based DPI technology. The event takes place at the Hotel Sonne and Harro Höfliger Verpackungsmaschinen GmbH headquarters in Germany. Mark Copley’s presentation will provide a basic introduction to the dose uniformity and aerodynamic particle size distribution methods required for the testing of orally inhaled products (OIPs), according to the requirements of... Breathing simulators are machines that mimic the inhalation and/or exhalation profile of a patient and are used increasingly in OIP testing. Copley Scientific’s white paper clearly explains where breathing simulators are specified by pharmacopoeial methods and also where their optional use is beneficial. This may be in improving understanding of product performance, for example, or to support a Quality by Design (QbD) approach...
Breathing simulators are machines that mimic the inhalation and/or exhalation profile of a patient and are used increasingly in OIP testing. Copley Scientific’s white paper clearly explains where breathing simulators are specified by pharmacopoeial methods and also where their optional use is beneficial. This may be in improving understanding of product performance, for example, or to support a Quality by Design (QbD) approach...
 The new unit frees up bench-space and simplifies connection of the key pieces of equipment routinely used for DPI testing, without compromising the validity of existing test methods. Measuring the aerodynamic particle size distribution of DPIs helps to determine their in vivo behaviour and is essential during product development and for QC. Cascade impaction is the technique specified by the regulators, and the Next Generation Impactor (NGI) is routinely the instrument of choice for this application. Testing must be carried out at a constant, controlled air flow rate to ensure that the NGI delivers precise, calibrated performance...
The new unit frees up bench-space and simplifies connection of the key pieces of equipment routinely used for DPI testing, without compromising the validity of existing test methods. Measuring the aerodynamic particle size distribution of DPIs helps to determine their in vivo behaviour and is essential during product development and for QC. Cascade impaction is the technique specified by the regulators, and the Next Generation Impactor (NGI) is routinely the instrument of choice for this application. Testing must be carried out at a constant, controlled air flow rate to ensure that the NGI delivers precise, calibrated performance...

 The powerful, oil lubricated SCP5 generates flow rates up to 100L/min (the limit required for all DPI testing) under the stable, sonic flow conditions necessary for robust aerodynamic particle size distribution (APSD) measurement, when used with all the pharmacopoeial specified cascade impactors. A quiet and space-efficient option for high flow rate generation, the SCP5 incorporates a flow control valve and regulated inlets that also enable testing of other types of orally inhaled and nasal drug products (OINDPs), for maximum flexibility...
The powerful, oil lubricated SCP5 generates flow rates up to 100L/min (the limit required for all DPI testing) under the stable, sonic flow conditions necessary for robust aerodynamic particle size distribution (APSD) measurement, when used with all the pharmacopoeial specified cascade impactors. A quiet and space-efficient option for high flow rate generation, the SCP5 incorporates a flow control valve and regulated inlets that also enable testing of other types of orally inhaled and nasal drug products (OINDPs), for maximum flexibility... Designed to allow closer simulation of in vivo breathing profiles, the BRS 3000 generates flow rates up to 240 litres per minute with a maximum 25 litres per second2 acceleration rate; volume capacity is 5 litres. Companion to the smaller BRS 2000 unit, which is designed principally for nebulisers, the BRS 3000 allows users to precisely define and apply breathing profiles that mimic those of the patient during inhaler use...
Designed to allow closer simulation of in vivo breathing profiles, the BRS 3000 generates flow rates up to 240 litres per minute with a maximum 25 litres per second2 acceleration rate; volume capacity is 5 litres. Companion to the smaller BRS 2000 unit, which is designed principally for nebulisers, the BRS 3000 allows users to precisely define and apply breathing profiles that mimic those of the patient during inhaler use... Visitors to the Copley Scientific stand at the 2012 European Aerosol Conference in Granada, Spain (2-7 September) will be able to find out more about new technology for the study of aerosol behaviour, as well as viewing the company's extensive range of aerosol sampling and particle characterisation equipment for pharmaceutical, defence and nanotechnology applications and atmospheric studies...
Visitors to the Copley Scientific stand at the 2012 European Aerosol Conference in Granada, Spain (2-7 September) will be able to find out more about new technology for the study of aerosol behaviour, as well as viewing the company's extensive range of aerosol sampling and particle characterisation equipment for pharmaceutical, defence and nanotechnology applications and atmospheric studies... A ‘go to' resource for expert advice that is rooted in the latest regulatory guidance and pharmacopoeial monographs, the new brochure includes details of a cluster of novel products designed to deliver better in vitro in vivo relationships (IVIVRs) for all orally inhaled and nasal drug products (OINDPs)...
A ‘go to' resource for expert advice that is rooted in the latest regulatory guidance and pharmacopoeial monographs, the new brochure includes details of a cluster of novel products designed to deliver better in vitro in vivo relationships (IVIVRs) for all orally inhaled and nasal drug products (OINDPs)... In May 2012, Copley Scientific's distribution partners from around the world gathered at the company's headquarters in Nottingham, UK, to attend an intensive three-day training programme on all aspects of inhaler testing. Over the course of the meeting, participants were able to hone their knowledge and skills, learning not only about new products, but also the latest regulatory thinking and requirements...
In May 2012, Copley Scientific's distribution partners from around the world gathered at the company's headquarters in Nottingham, UK, to attend an intensive three-day training programme on all aspects of inhaler testing. Over the course of the meeting, participants were able to hone their knowledge and skills, learning not only about new products, but also the latest regulatory thinking and requirements... Building on an established reputation as a global provider of high quality pharmaceutical testing equipment, Copley Scientific is significantly expanding its world-wide distribution network with the appointment of new partners in Turkey, Brazil and China. "Copley Scientific aims to provide the very highest levels of localised service to all its customers, around the world," said Sales Director Mark Copley, "so we're delighted to have identified strong distribution partners for these three important territories. The economies of Turkey, Brazil and China are flourishing, with high levels of pharmaceutical research activity taking place...
Building on an established reputation as a global provider of high quality pharmaceutical testing equipment, Copley Scientific is significantly expanding its world-wide distribution network with the appointment of new partners in Turkey, Brazil and China. "Copley Scientific aims to provide the very highest levels of localised service to all its customers, around the world," said Sales Director Mark Copley, "so we're delighted to have identified strong distribution partners for these three important territories. The economies of Turkey, Brazil and China are flourishing, with high levels of pharmaceutical research activity taking place... Visitors to Respiratory Drug Delivery 2012 (RDD 2012, May 13-17, Phoenix, Arizona) will be the first to see the new Copley Scientific BRS 3000 breath simulator for metered dose inhaler (MDI) and dry powder inhaler (DPI) testing. Companion to the BRS 2000 unit for nebulisers, which was previewed at ISAM 2011, the BRS 3000 allows users to precisely define and apply breathing profiles that mimic those of the patient during inhaler use. This enables the exploration of DPI and MDI performance under test conditions that more accurately reflect in-use situations...
Visitors to Respiratory Drug Delivery 2012 (RDD 2012, May 13-17, Phoenix, Arizona) will be the first to see the new Copley Scientific BRS 3000 breath simulator for metered dose inhaler (MDI) and dry powder inhaler (DPI) testing. Companion to the BRS 2000 unit for nebulisers, which was previewed at ISAM 2011, the BRS 3000 allows users to precisely define and apply breathing profiles that mimic those of the patient during inhaler use. This enables the exploration of DPI and MDI performance under test conditions that more accurately reflect in-use situations... Copley Scientific is exhibiting its market leading inhaled product testing systems at Analytica 2012 (17-20 April; Munich, Germany), together with instruments from the company's comprehensive range of solid dosage testing equipment. Inhaler testing systems from Copley Scientific are used throughout the pharmaceutical industry and are supported by a team with specialist knowledge and expertise in this area...
Copley Scientific is exhibiting its market leading inhaled product testing systems at Analytica 2012 (17-20 April; Munich, Germany), together with instruments from the company's comprehensive range of solid dosage testing equipment. Inhaler testing systems from Copley Scientific are used throughout the pharmaceutical industry and are supported by a team with specialist knowledge and expertise in this area... Copley Scientific, a successful Nottinghamshire company that supplies test equipment to pharmaceutical companies across the globe , is taking a keen interest in activities much closer to home, signing a sponsorship agreement with local under 13's soccer side Edwalton Cavaliers...
Copley Scientific, a successful Nottinghamshire company that supplies test equipment to pharmaceutical companies across the globe , is taking a keen interest in activities much closer to home, signing a sponsorship agreement with local under 13's soccer side Edwalton Cavaliers... The recent European Aerosol conference (EAC) (4-9 September 2011) in Manchester saw the launch of a new series of compact, environmentally friendly water-based condensation particle counters (WCPC) from Copley Scientific. Suitable for use in clean rooms, environmental science applications, high purity gas systems, and other air sampling applications, the M1110 and the M1120 WCPCs use novel single-flow mixing technology to give superior counting at high particle concentrations...
The recent European Aerosol conference (EAC) (4-9 September 2011) in Manchester saw the launch of a new series of compact, environmentally friendly water-based condensation particle counters (WCPC) from Copley Scientific. Suitable for use in clean rooms, environmental science applications, high purity gas systems, and other air sampling applications, the M1110 and the M1120 WCPCs use novel single-flow mixing technology to give superior counting at high particle concentrations... Returning from a successful trip to China, where he was the invited guest of the National Institute for Food and Drug Control (NIFDC) (the department of the State Food and Drug Adminstration (SFDA) responsible for drug testing) Mark Copley, Sales Director for Copley Scientific, is enthusiastic about how inhaled product testing is developing in the country and delighted with the response to training sessions that he delivered...
Returning from a successful trip to China, where he was the invited guest of the National Institute for Food and Drug Control (NIFDC) (the department of the State Food and Drug Adminstration (SFDA) responsible for drug testing) Mark Copley, Sales Director for Copley Scientific, is enthusiastic about how inhaled product testing is developing in the country and delighted with the response to training sessions that he delivered... Copley Scientific is expanding the team at its Nottingham, UK, headquarters, most recently adding a new product support engineer, to meet increasing demands from its rapidly growing international business. At the same time the company has successfully extended its ISO certification to include the equipment design process, further enhancing its credentials to also offer bespoke technical solutions for the pharmaceutical industry...
Copley Scientific is expanding the team at its Nottingham, UK, headquarters, most recently adding a new product support engineer, to meet increasing demands from its rapidly growing international business. At the same time the company has successfully extended its ISO certification to include the equipment design process, further enhancing its credentials to also offer bespoke technical solutions for the pharmaceutical industry... The 2011 European Aerosol Conference in Manchester, UK (4-9 September 2011) will see Copley Scientific exhibiting the company's latest solutions for aerosol sampling and particle characterisation. Copley Scientific offers instrumentation for aerosol research in a number of important areas including pharmaceuticals, atmospheric studies, defence and nanotechnology. A particular highlight at the show will be the Real-Time Fibre Monitor Model 7400AD, a compact and powerful system that is being adopted for a range of applications...
The 2011 European Aerosol Conference in Manchester, UK (4-9 September 2011) will see Copley Scientific exhibiting the company's latest solutions for aerosol sampling and particle characterisation. Copley Scientific offers instrumentation for aerosol research in a number of important areas including pharmaceuticals, atmospheric studies, defence and nanotechnology. A particular highlight at the show will be the Real-Time Fibre Monitor Model 7400AD, a compact and powerful system that is being adopted for a range of applications... Copley Scientific will exhibit the Tergotometer detergent testing system at CESIO 2011, the 8th World Surfactant Congress and Business Convention, which takes place in Vienna, Austria from June 6 - 8, 2011. The Copley Scientific Tergotometer is a convenient and economical laboratory-scale system that is widely used to test the effectiveness of soaps and detergents or the washability and colourfastness of fabrics and other materials...
Copley Scientific will exhibit the Tergotometer detergent testing system at CESIO 2011, the 8th World Surfactant Congress and Business Convention, which takes place in Vienna, Austria from June 6 - 8, 2011. The Copley Scientific Tergotometer is a convenient and economical laboratory-scale system that is widely used to test the effectiveness of soaps and detergents or the washability and colourfastness of fabrics and other materials... Copley Scientific has delivered a multiple order of eight tablet dissolution testers to Melbourn Scientific as the contract research organisation extends its testing capabilities in response to increasing client demand. The eight DIS6000 systems, one of the most compact dissolution testers available, have been installed and commissioned with full support from the Copley Scientific team who completed installation and operation qualification (IQ/OQ) on site...
Copley Scientific has delivered a multiple order of eight tablet dissolution testers to Melbourn Scientific as the contract research organisation extends its testing capabilities in response to increasing client demand. The eight DIS6000 systems, one of the most compact dissolution testers available, have been installed and commissioned with full support from the Copley Scientific team who completed installation and operation qualification (IQ/OQ) on site... Current interest in the use of Abbreviated Impactor Measurement (AIM) was clear at the recent EPAG (European Pharmaceutical Aerosol Group) sponsored workshop at Drug Delivery to the Lungs 21, where presenters from a number of leading pharmaceutical companies shared experimental data comparing AIM results. New instrumentation, such as the Fast Screening Impactor (FSI) and Fast Screening Andersen (FSA), from Copley Scientific, were shown to deliver data comparable to conventional cascade impaction in less time
Current interest in the use of Abbreviated Impactor Measurement (AIM) was clear at the recent EPAG (European Pharmaceutical Aerosol Group) sponsored workshop at Drug Delivery to the Lungs 21, where presenters from a number of leading pharmaceutical companies shared experimental data comparing AIM results. New instrumentation, such as the Fast Screening Impactor (FSI) and Fast Screening Andersen (FSA), from Copley Scientific, were shown to deliver data comparable to conventional cascade impaction in less time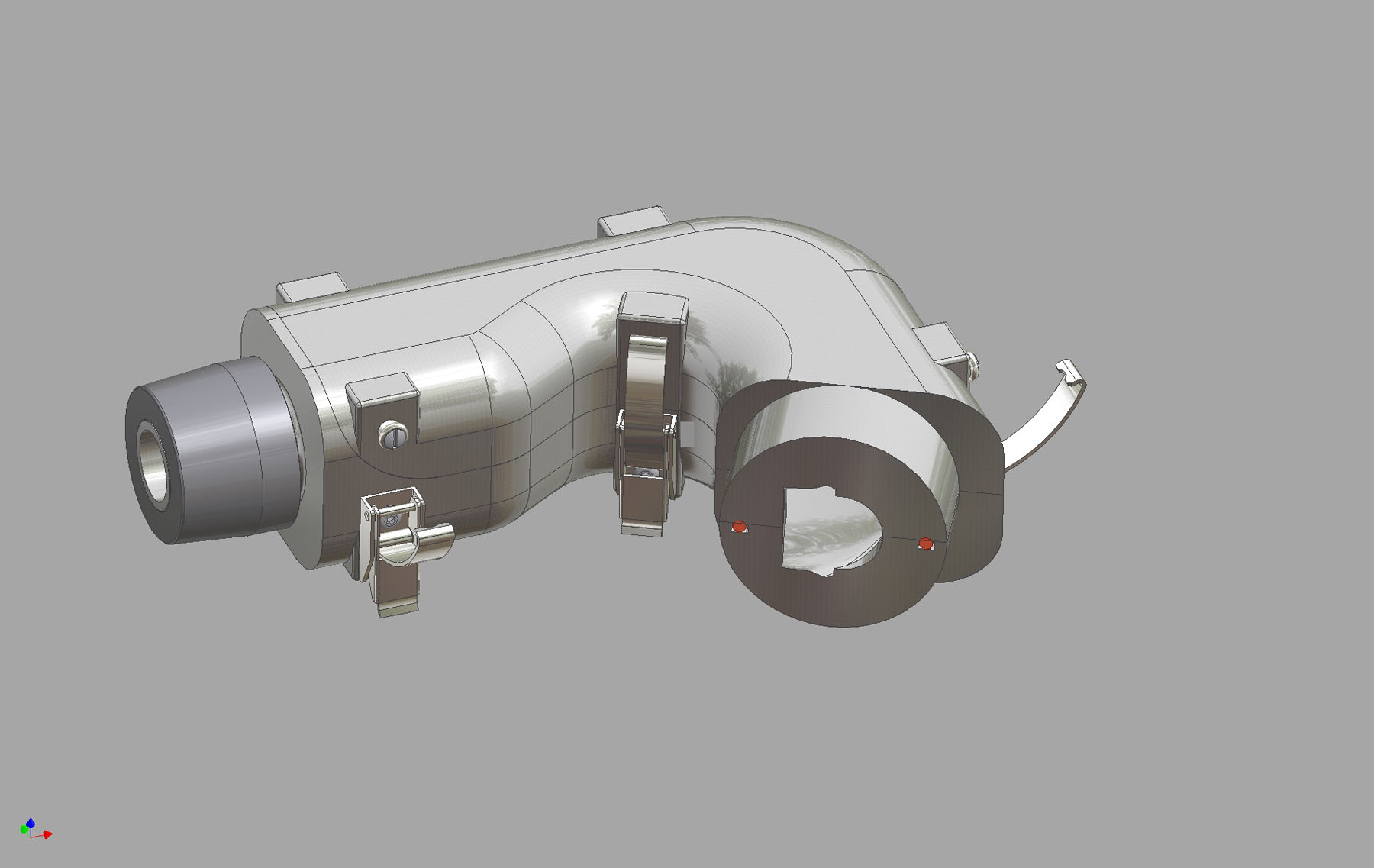 The Alberta Idealized Throat is a new product from Copley Scientific designed to improve in vivo/in vitro correlation (IVIVC) during inhaled product testing. Originally developed by the University of Alberta, Canada, and commercialized exclusively by Copley Scientific, the new throat is designed to provide a more ‘patient representative' alternative to the USP/Ph Eur induction port, routinely used for aerodynamic particle size measurement by cascade impaction.
The Alberta Idealized Throat is a new product from Copley Scientific designed to improve in vivo/in vitro correlation (IVIVC) during inhaled product testing. Originally developed by the University of Alberta, Canada, and commercialized exclusively by Copley Scientific, the new throat is designed to provide a more ‘patient representative' alternative to the USP/Ph Eur induction port, routinely used for aerodynamic particle size measurement by cascade impaction. At this year's Drug Delivery to the Lungs conference, Copley Scientific will launch the Alberta Idealized Throat, a new induction port designed for use with with cascade impactors to improve the in vivo relevance of in vitro inhaled product testing. DDL21 takes place from 8 - 10 December in Edinburgh, Scotland.
At this year's Drug Delivery to the Lungs conference, Copley Scientific will launch the Alberta Idealized Throat, a new induction port designed for use with with cascade impactors to improve the in vivo relevance of in vitro inhaled product testing. DDL21 takes place from 8 - 10 December in Edinburgh, Scotland.